黄葵胶囊由苏中药业集团股份有限公司生产,于1999年获得中国国家药品监督管理局批准(批准号:Z19990040),用于治疗肾脏疾病(包括糖尿病肾病),其主要活性成分为黄蜀葵花总黄酮[1]。该药物是一种口服中成药,顾丰教授与吴洁、唐伟(南京医科大学附属老年医院)、强磊教授合作,分别采用1型糖尿病(NOD)和2型糖尿病(db/db)小鼠模型,系统探究黄葵胶囊及其总黄酮对肠肾轴的调控作用。研究结果表明该药物可通过改善肠道微生态平衡及调节代谢物水平,下调关键的细胞溶质递体表达,进而发挥肾脏保护作用[2-4]。同时,顾丰教授与省中医院孙伟教授团队合作探究黄葵胶囊与厄贝沙坦联用的临床价值[5],并通过动物实验进一步揭示其协同保护肾脏的作用机制[6]。此外,与孙建国教授合作采用代谢物鉴定,系统分析了黄葵胶囊及其总黄酮在db/db小鼠体内代谢的动态变化,为深入阐明黄葵胶囊总黄酮治疗糖尿病肾病的药效物质基础提供了依据[7],并揭示了其通过调控肾上腺类固醇激素合成及肾醛固酮-盐皮质激素受体信号通路发挥肾脏保护作用的分子机制[8]。顾丰教授团队进一步采用单细胞转录组技术分析黄葵总黄酮治疗糖尿病肾病的作用机制[9]。最近以题为“Single-Cell Spatial Transcriptomics Reveals Potential Molecular Mechanisms of Abelmoschus manihot (L.) Medic in Treating Diabetic Kidney Disease”的论文发表于iMeta杂志。第一作者为吴晨华博士,通信作者为顾丰、吴洁和唐海涛。这项研究将分辨率为0.25微米的空间转录组测序与单细胞全长RNA测序技术相结合,从转录组调控的角度,构建了厄贝沙坦与黄葵总黄酮治疗糖尿病肾病的调控网络,并通过全转录组组基因筛选和分子对接模拟,鉴定出黄葵总黄酮作用于肾脏细胞的关键受体(如Itga3、Itga5、Tgfbr1等)和调控因子(如Jun、Junb、Stat1等),为黄葵胶囊及其主要化学成分治疗糖尿病肾病的分子机制提供了新的见解。
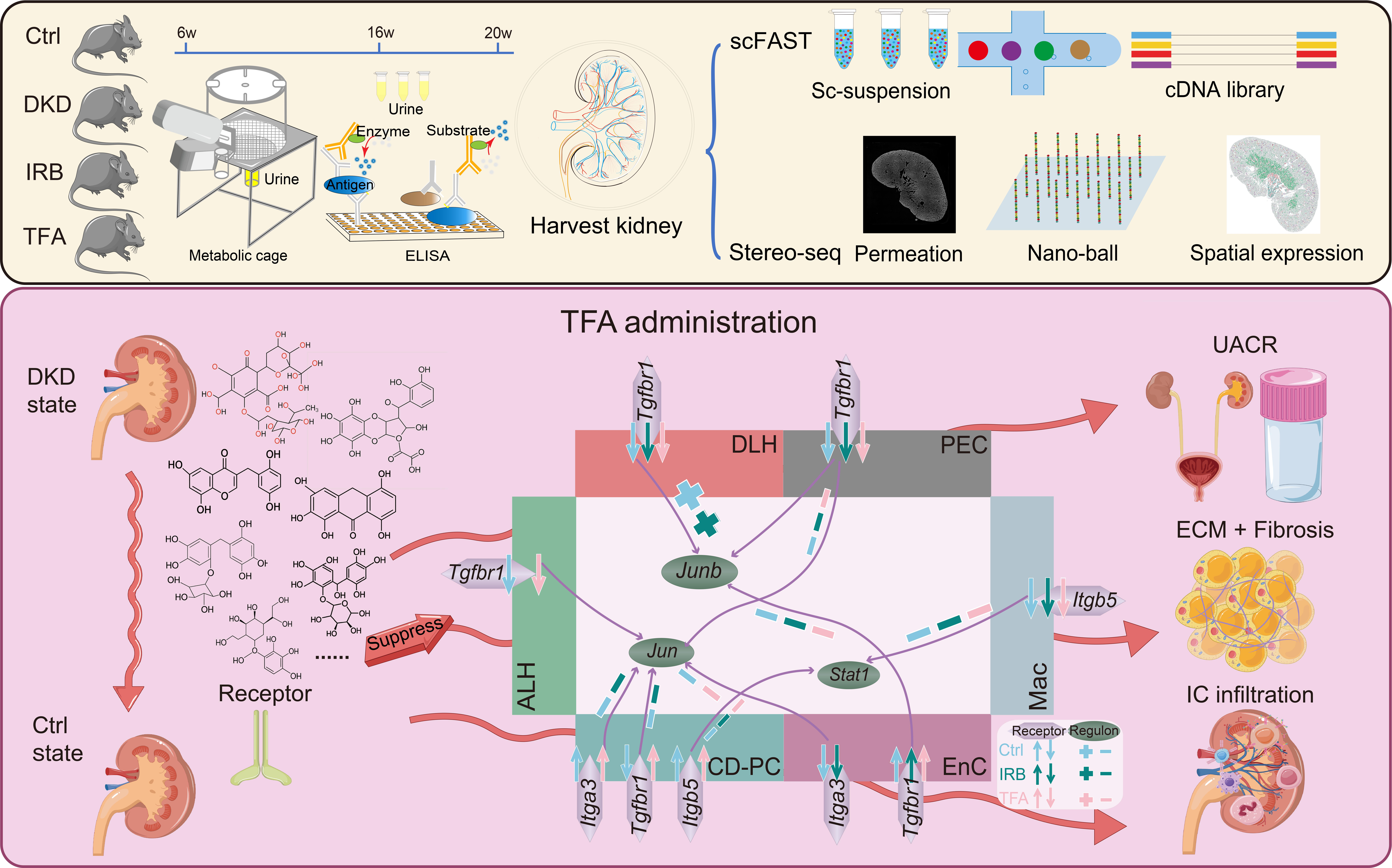
发表的相关论文
1 Li N, Tang H, Wu L, Ge H, Wang Y, Yu H, Zhang X, Ma J, Gu HF*. Chemical constituents, clinical efficacy and molecular mechanisms of the ethanol extract of Abelmoschus manihot flowers in treatment of kidney diseases. Phytother Res. 2021;35(1):198-206. doi: 10.1002/ptr.6818. Q1/6.3
2 Shi R, Tao Y, Tang H, Wu C, Fei J, Ge H, Gu HF*, Wu J*. Abelmoschus Manihot ameliorates the levels of circulating metabolites in diabetic nephropathy by modulating gut microbiota in non-obese diabetes mice. Microb Biotechnol. 2023;16(4):813-826. doi: 10.1111/1751-7915.14200. Q1/5.2
3 Yu H, Tang H, Wang M, Xu Q, Yu J, Ge H, Qiang L, Tang W*, Gu HF*. Effects of total flavones of Abelmoschus manihot (L.) on the treatment of diabetic nephropathy via the activation of solute carriers in renal tubular epithelial cells. Biomed Pharmacother. 2023;169:115899. doi: 10.1016/j.biopha.2023.115899. Q1/7.5
4 Yu H, Wang M, Yu J, Tang H, Xu Q, Cheng N, Luo X, Wang Y, Ge H, Qiang L*, Tang W*, Gu HF*. Evaluation of the efficacy of Abelmoschus manihot (L.) on diabetic nephropathy by analyzing biomarkers in the glomeruli and proximal and distal convoluted tubules of the kidneys. Front Pharmacol. 2023;14:1215996. doi: 10.3389/fphar.2023.1215996. Q1/4.8
5 Zhao J, Tostivint I, Xu L, Huang J, Gambotti L, Boffa JJ, Yang M, Wang L, Sun Z, Chen X, Liou-Schischmanoff A, Baumelou A, Ma T, Lu G, Li L, Chen D, Piéroni L, Liu B, Qin X, He W, Wang Y, Gu HF, Sun W. Efficacy of Combined Abelmoschus manihot and Irbesartan for Reduction of Albuminuria in Patients with Type 2 Diabetes and Diabetic Kidney Disease: A Multicenter Randomized Double-Blind Parallel Controlled Clinical Trial. Diabetes Care. 2022;45(7):e113-e115. doi: 10.2337/dc22-0607. Q1/16.6
6 Yu H, Tang H, Saxu R, Song Y, Cui X, Xu J, Li N, Cui S, Ge H, Tang W*, Gu HF*. Effects of Abelmoschus manihot (L.) and its combination with irbesartan in the treatment of diabetic nephropathy via the gut-kidney axis. Front Pharmacol. 2024;15:1424968. doi: 10.3389/fphar.2024.1424968. Q1/4.8
7 Diao Z, Yu H, Wu Y, Sun Y, Tang H, Wang M, Li N, Ge H, Sun J*, Gu HF*. Identification of the main flavonoids of Abelmoschus manihot (L.) medik and their metabolites in the treatment of diabetic nephropathy. Front Pharmacol. 2024;14:1290868. doi: 10.3389/fphar.2023.1290868. Q1/4.8
8 Saxu R, Tang H, Yu H, Ge H, Gu HF*. Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, inhibits adrenal aldosterone synthesis and renal ERK/EGR1 pathway in the treatment of diabetic kidney disease. J Ethnopharmacol. 2025;349:119936. doi: 10.1016/j.jep.2025.119936. Q1/5.4
9 Wu C, Tang H, Cui X, Li N, Fei J, Ge H, Wu L, Wu J*, Gu HF*. A single-cell profile reveals the transcriptional regulation responded for Abelmoschus manihot (L.) treatment in diabetic kidney disease. Phytomedicine. 2024;130:155642. doi: 10.1016/j.phymed.2024.155642. Q1/8.3
10 Wu C, Tang H*, Yu Y, Song Y, Shen Y, He H, Wu J*, Gu HF*. Single-Cell Spatial Transcriptomics Reveals Potential Molecular Mechanisms of Abelmoschus manihot (L.) Medic in Treating Diabetic Kidney Disease. iMeta. 2025;4:e70099. doi: 10.1002/imt2.70099. Q1/33.2
(撰稿人:余怡虹,李宇鹏)